Exploration of Non-systemic Cancer Treatment Modalities
Globally, 18.1 million new cases of cancer and 9.6 million cancer-related deaths have been estimated for 2018 [1]. With the number of people worldwide diagnosed with cancer rapidly growing, there is much effort in aiming to curtail cancer progression. All sectors of the cancer community, from research to care, are actively working towards meeting this goal through discovery of novel interventions and their application in cancer care settings.
The type and stage of cancer, along with the needs and wants of cancer patients inform the trajectory of care. Some patients may have to go through a combination of treatments, and interact with more than one oncologist, in order to destroy or manage the tumor cancer cells. As mentioned in our last article, there are three main branches of oncology that consist of systemic and non-systemic cancer therapy regimens. Medical oncology treatment measures fall under systemic treatments, where substances travel through the bloodstream in order to reach and affect cells all over the body. Surgical and radiation oncology involve non-systemic therapies, where surgical and radiation oncologists lead the healthcare team through the informing, planning, and administration phases of care. We will continue by exploring the fields of surgical and radiation oncology.
Within cancer therapies, surgery was the primal treatment method where innovative surgeons pushed boundaries to find a cure. Typically the cancers amenable to surgical treatment are called solid organ cancers, for instance, cancers involving the mouth, intestinal system, breast, brain and bone. In nearly all of these cases, a surgical oncologist performs surgery to obtain a diagnosis or get rid of the tumor mass. This therapy can be used alone, or in combination with other treatment methods. To this day, surgery remains a promising cancer therapy modality as new technologies have supported more precision in surgical care. Some of the latest advances which have been incorporated into practice are:
1) Organ preservation surgery – This form of treatment is performed in combination with radiation and/or chemotherapy to shrink tumors. This method allows for a less mutilating but equally effective operation. A usage of this method has been for rectal cancers. In the past, a colostomy was needed for patients with rectal cancers that resulted in a complete removal of the colon and the placement of a permanent bag to drain intestinal content. Now, oncologists are able to shrink tumors with radiation or chemotherapy, leading to a majority of patients having a normal intestinal passageway.
2) Laparoscopic surgery – Cancer surgery used to regularly involve open large incisions. However, numerous studies have shown that surgery using laparoscopic techniques is equally as effective, has similar cure rates, is less painful and has a quicker recovery period when compared to open surgery [2]. Laparoscopic surgery is a specialized technique that uses several 0.5 – 1 cm incisions, called a “port”, to insert specialized instruments and a camera to allow the surgeon to perform the
same operations as traditional surgery but with smaller incisions. Laparoscopic surgery is routinely offered for cancers of the esophagus, stomach, large intestine and for gynecologic cancers (uterus and cervix).
3) Robotic surgery – This novel technology assists oncology surgeons through a computer interface to perform meticulous surgeries, particularly for abdominal and chest cancers. Through a small incision, the surgeon performs the surgery by sitting at a console that controls the robotic instrument inside the patient. The surgeon has complete control over the robot. The advantage of this robotic technology is that it allows surgeons to reach the narrowest of spaces to clear the tumor, resulting in better visibility and preservation of nerves and critical structures. In addition, less blood is lost by the patient and their recovery time is reduced, when compared to laparoscopy an open surgery. As a newer from of minimally invasive surgery, its costs, limited availability, and need for specialized training are current barriers.
Radiation therapy is an important mode of therapy and treatment, where approximately 50% of cancer patients receive radiation therapy in their cancer care continuum and contributes to 40% of all therapeutic treatments for cancer. The main goal of radiation therapy is to kill cancer cells through targeted radiation beams. It aims to reduce the number of healthy cells that are lost during treatment, when compared to systemic therapies, by only targeting the areas of the body affected by cancer. High-energy radiation damages the genetic material of cells, which causes cell death. Unfortunately, the radiation still affects the healthy cells surrounding cancer cells, but the healthy cells have the ability to heal themselves at a faster rate following treatment and retain normal functioning.
The birth of radiation therapy took place in 1898, when Marie Curie discovered radium as a source of radiations [3]. Through the years, radiation therapy has evolved. Ongoing advances in techniques and in understanding the biology of cancer cell responses to radiation have led to increased survival and reduced treatment side effects for cancer patients.
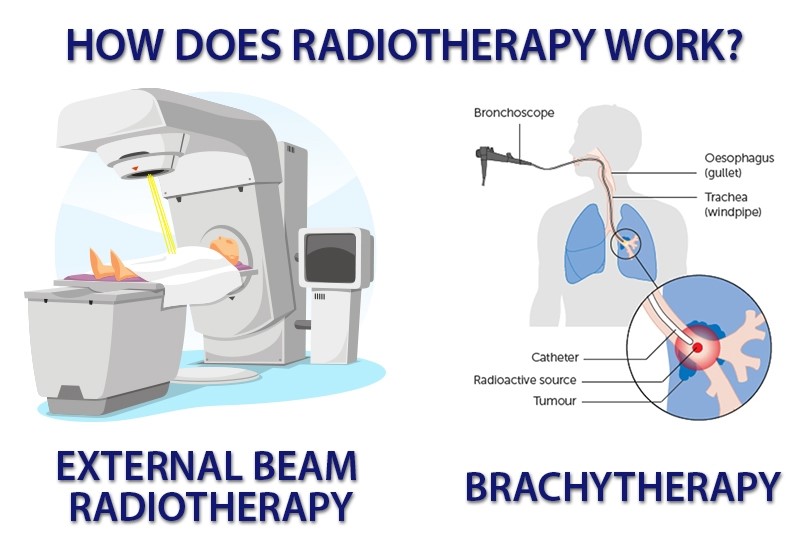
Currently, there are two ways of delivering radiation to the cancer site:
1) External beam radiation is delivered from outside the body by aiming high-energy rays, such as photons, protons or particle radiation, to the tumor site. This method is currently the most common approach in the clinical setting.
2) Internal radiation or brachytherapy [4] is delivered from inside the body by a radioactive implant, sometimes called a seed or capsule. This form of delivery allows a higher dose of radiation in a smaller area that might be feasible with external radiation treatment.
It is technological advances, such as new imaging modalities, more powerful computers, and new delivery systems, that have helped advanced the goal of radiotherapy [5].
Some of these advances include:
3D conformal radiotherapy (3DCRT) is an imaging technique that allows accurate localization of the tumor and critical normal organ structures. 3DCRT allows for optimal beam placement and shielding.
Intensity-modulated radiation therapy (IMRT) is an advanced mode of high-precision radiotherapy that uses computer-controlled linear accelerators to deliver precise radiation doses to a malignant tumor or specific areas within the tumor. IMRT allows radiation oncologists to deliver radiation dosages that conform more precisely to the three-dimensional shape of the tumor by controlling the intensity of the radiation beam.
Image-guided radiotherapy (IGRT) uses X-rays and scans to identify the size, shape, and position of the cancer as well as the surrounding tissues and bones. This advancement is beneficial when treatment margins become tighter and more conformal as a positional error would lead to inadvertent radiation of normal healthy organs. IGRT eliminates this unintended consequence as it obtains accurate information through pre-radiotherapy imaging.
Stereotactic body radiation therapy (SBRT) delivers very high individual doses of radiation from many different positions around the patient’s body, while meeting at the tumor. In turn, the tumor receives a high dose of radiation and the tissues surrounding it receive a low dose. SBRT has shown excellent results in the treatment of early stage non-small cell lung cancer in patients unfit for surgery. Other tumors include in the prostate, head and neck, hepatic, renal, spinal and pancreatic.
Proton beam therapy, also called proton therapy, uses a machine that speeds up protons. These high-speed protons create high energy that gives the targeted radiation dose in the tumor. Proton therapy is becoming more prevalent as it is the most advanced form of radiation available [5]. This method of treatment eliminates exit radiation, so radiation oncologists are able to increase dosage to more powerfully and precisely target tumors. Clinical studies have shown that proton therapy has proven to be a cost-effective treatment for pediatric brain tumor patients [6].
Without a doubt, it is an exciting time for oncology. Rapid progress in this field continues to be boosted by technological advances. While mentioned, and more, treatments methods have already transformed cancer care, the growing understanding of the biology of cancer will take therapy to an entirely new level in the coming decades. The hope is for these tools to address the complexity of cancer – the combination of patient-specific characteristics that drive the development of each person’s disease, response to therapy and long-term toxicities. We look forward to tracking and learning more about these advances, and the dynamic interplay between the three pillars of oncology: medical oncology, surgical oncology, and radiation oncology.
Written by Dorri Mahdaviani , who holds a Masters of Public Health (MPH) from the University of British Columbia (UBC). Her academic and professional interests include the areas of chronic illnesses, health care systems and childhood health and development.