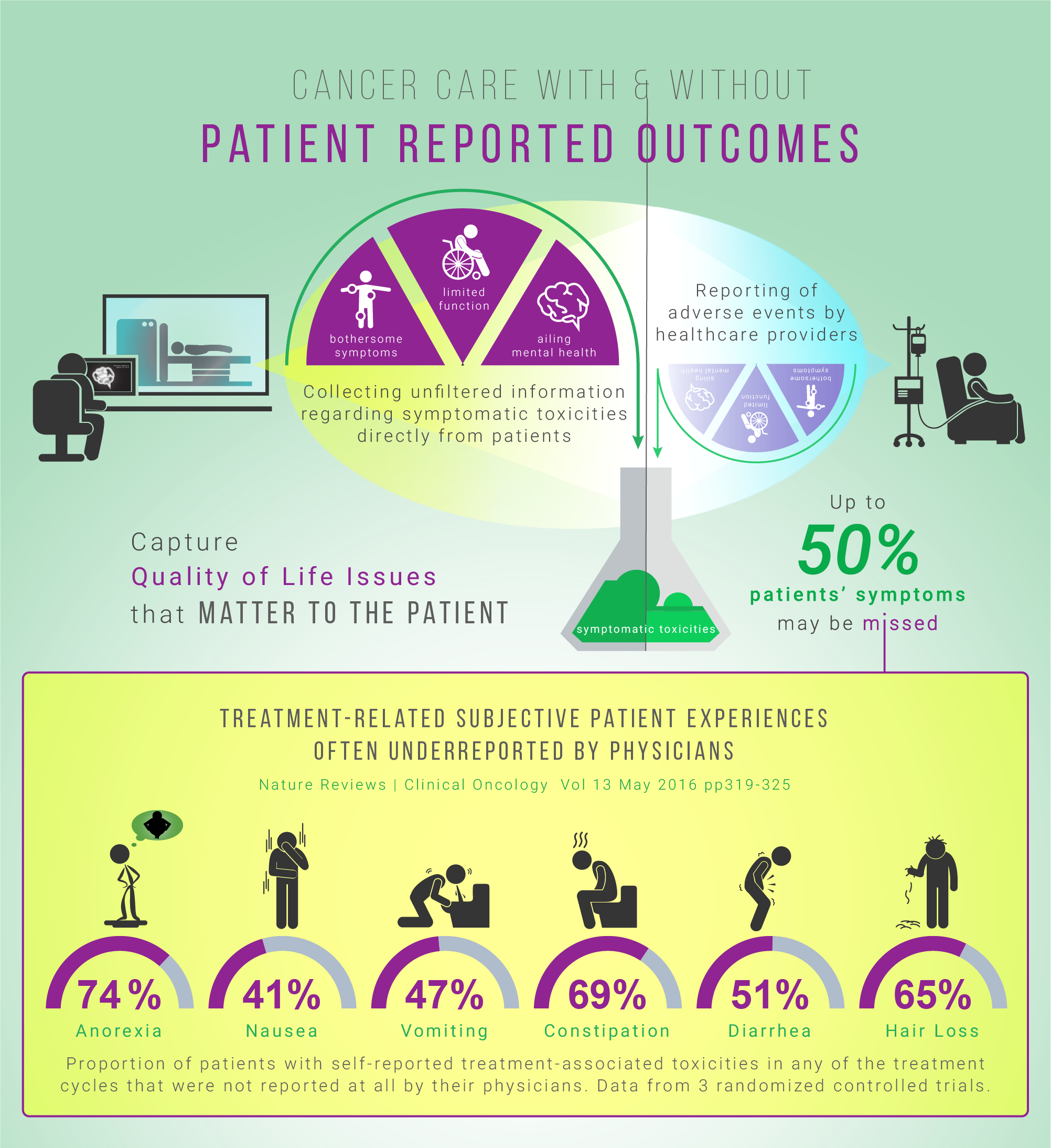
In a given year, over 1.5 million new cases of cancer are diagnosed in the United States alone [1]. Their journey on the cancer care continuum starts with screening and continues through survivorship, getting matched with the most appropriate form of care. Radiation, chemo, or hormonal therapy as well as surgery, all have side-effects that are experienced first-hand by the patient and impact their families.
Currently, traditional biomedical outcome measures, such as survival and disease-free survival rates, are widely used to track the effectiveness of cancer treatment interventions [2]. Symptomatic toxicities are often underreported when collected by healthcare professionals [3]. In a chemo radiation cancer study, comparing patient-reported outcomes (PROs) with hospital charts of acute symptoms, the patients reported diarrhea and inflammation approximately 20% more often than clinicians [4]. Patients generally report symptoms earlier and more frequently than clinicians. They are also able and willing to self-report their own adverse symptoms, even when severely ill. [5]
In one clinical trial, it was concluded that physician reporting is neither sensitive or specific in detecting common chemotherapy adverse effects [6]. To enhance current assessment measures, novel approaches to evaluating symptomatic toxicities associated with cancer treatments (e.g. headaches, nausea, vomiting) need to be incorporated into the health tracking systems. The aim is to better capture the rate of toxicity of interventions at a personalized level.
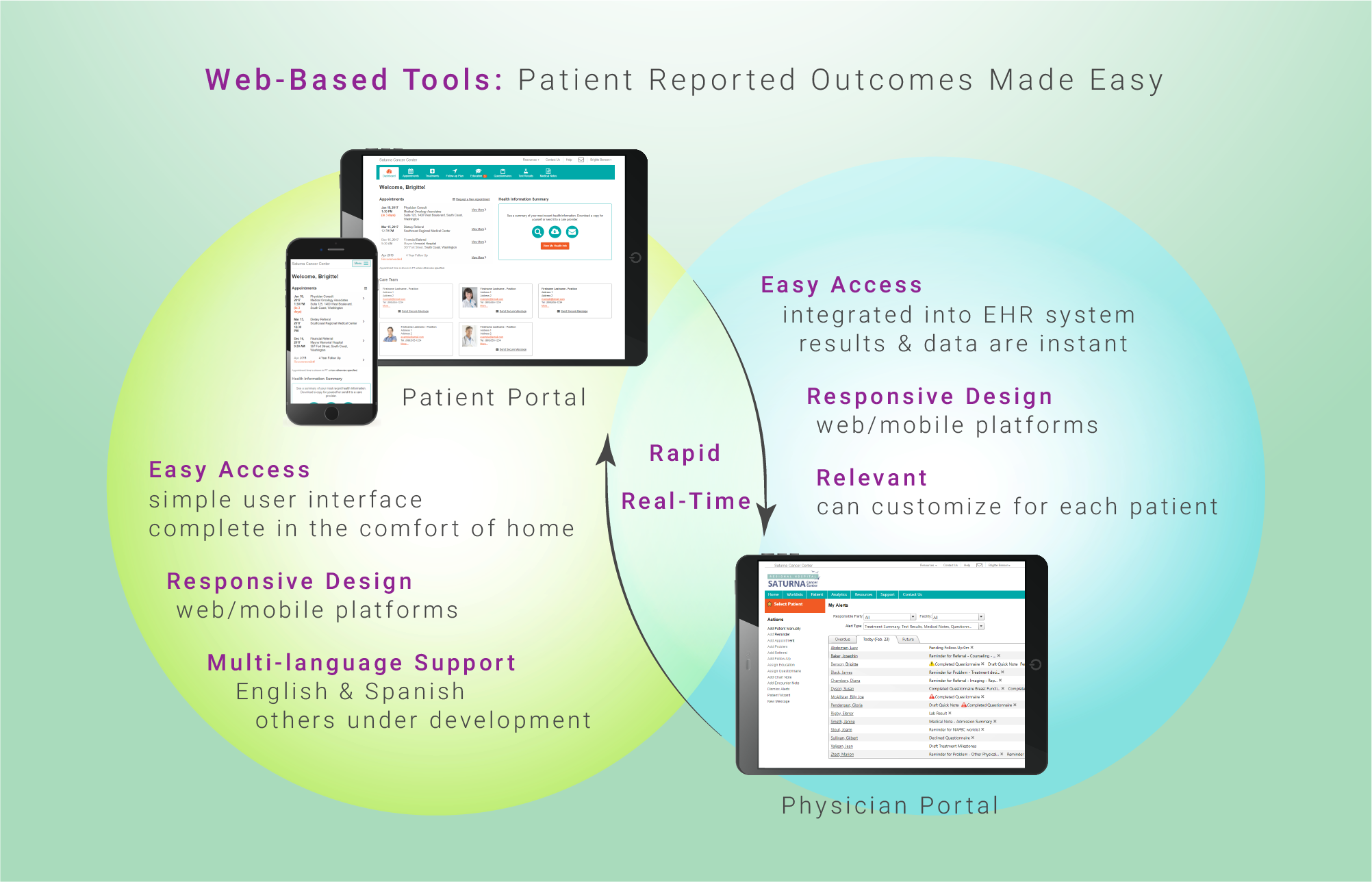
Direct patient reporting through electronic platforms offers a mechanism that will enhance the efficiency and precision of current approaches. Accurate knowledge of the potential harms and risks of cancer treatment modalities is important to decision makers including patients, researchers, health professionals, and regulatory authorities. Patient reported toxicities help guide healthcare professionals in real time in an effort to reduce treatment-related side effects. Patients are also better able to identify baseline symptoms related to pre-existing conditions, and misinterpretation or transformation of information is avoided. Undoubtedly, they will receive more accurate information based on their own reporting and previous experiences of their peers, and will be more at ease about the care provided. Tools developed that enable the translation of side effects, from the patient perspective, would allow tremendous improvements in risk-benefit analyses.
A systematic collection of patient-reported outcomes has been proven to be a valid, reliable, and sustainable approach in obtaining symptomatic toxicities. E-platforms have the ability to positively complement clinician toxicity reports. The widespread adoption of patient-reported outcome toxicity monitoring in oncology care is on the rise. Companies including Equicare Health try to address the need through comprehensive patient-reported outcome questionnaires. They aim to address the previously dominant challenges pertaining data collection logistics, validated analytical approaches, and resource utilization.
Written by Dorri Mahdaviani , who holds a Masters of Public Health (MPH) from the University of British Columbia (UBC). Her academic and professional interests include the areas of chronic illnesses, health care systems and childhood health and development.
Infographics and Design by Ann Wong, who holds a PhD in Biochemistry and Molecular Biology from the Faculty of Medicine at the University of British Columbia (UBC), Canada. She is an author of over 10 SCI publications, having taught at UBC and the Peking University Health Science Center (PUHSC) in Beijing