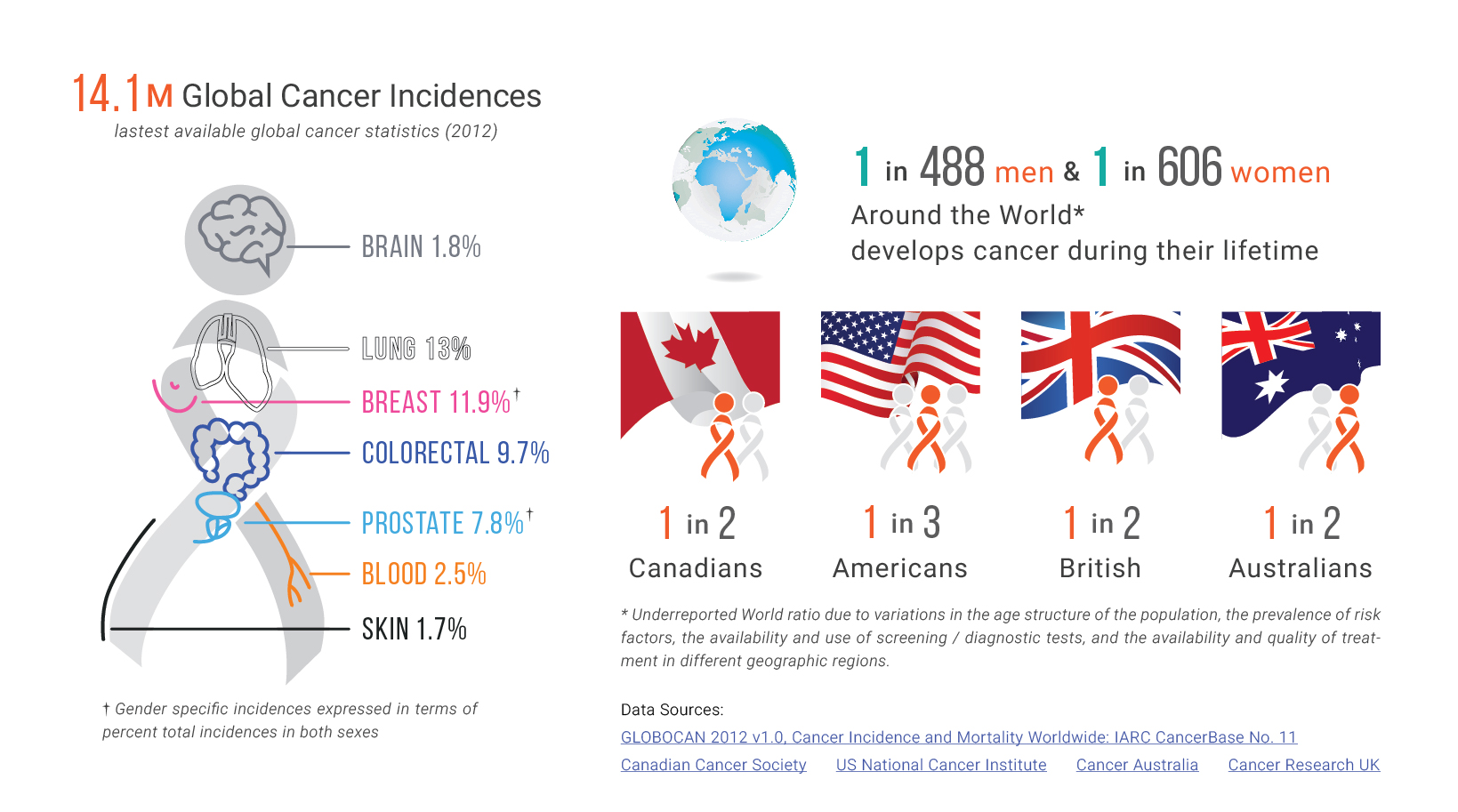
Cancer screening revolutionized the world of cancer care with its aim to detect cancer before symptoms appear. Early detection is paramount for the control of any disease. When symptoms are detected or apparent, diagnostic tests are used to find out the cause of the symptoms. A procedure, ranging in complexity and invasiveness, can have significant influences on detection, diagnosis, and trajectory of care. Once detected, engaging an oncologist early and connecting the screening results to the expertise needed enhances patient outcomes.
There are different cancer screening procedures in use across the range of cancer types. In the case of breast cancer and colorectal cancer, early detection is correlated with lowering mortality rates [1]. There are times that early detection does not lengthen the life of the patient, but may have other benefits with creating patient awareness. In general, screening tests are recommended and offered to all at-risk individuals, regardless of the outcome. This makes it imperative to be aware of the positive and negative impacts.
Two procedures that have been proven to be very effective at reducing cancer mortality rates are colorectal cancer and breast cancer screening. Colorectal cancer is the second leading cause [2] of cancer deaths in the United States. It mainly develops from abnormal growths in the colon or rectum. Screening tests can identify these abnormal growths. They can also detect tumors, and prevent the development of cancer before growths metastasize.
Interestingly, breast cancer mortality rates are lower than colorectal cancer rates. However, the rate of breast cancer diagnosis are higher within American women [3]. How is it that death rates are lower and diagnoses are higher for breast cancer, when compared to colorectal cancer? The answer is complex and depends on the screening tools used along with the nature of the cancer type and stage. Mammography is currently the most commonly supported screening procedure for breast cancer. Its greatest benefit is the decrease in breast cancer mortality: mammography screening has been associated with a 15% to 20% reduction in breast cancer mortality in women aged 40 to 74 years [4]. Despite the differences between colorectal and breast cancer, it is evident that timely and regular screening is key in lowering mortality rates.
An example of where early screening does not necessarily decrease mortality rates is the prostate-specific antigen (PSA) test for prostate cancer detection [5]. This blood test is able to detect prostate cancer at an early stage. However, routine PSA testing is debated. It is unclear whether early detection and treatment lead to significant changes in the cancer trajectory and the patient’s quality of life. The inadequate evidence of benefits for PSA testing hinders support for this screening test, and shines light on some unintentional consequences.
Recognizing the diversity of cancer screening modalities allows us to understand the scope of their influence across the cancer care spectrum. All tests are followed by results, and cancer screening is no exception. The test has either identified cancer in the individual, or it has not. However, there are truly four outcomes:
| True Negative | The screening test says the patient does not have cancer when they do not truly have cancer |
| False Positive | The screening test says the patient has cancer when they do not truly have it |
| False Negative | The screening test was not able to detect cancer when the patient truly has cancer |
| True Positive | The screening test says the patient has cancer and the patient truly has cancer |
In the True Negative scenario (an ideal case as a patient is cancer free), the potential implications from the screening test are procedural and financial. For example, when screening for colorectal cancer through colonoscopy, perforation and bleeding may occur.
For False Positive and Negative results, the screening test was not able to accurately identify the true state of the disease. When screening results appear abnormal even though there is no cancer (False Positive), anxiety may arise and each additional procedure may pose its own risks. For breast cancer, on average, 10% of women will be recalled from each screening examination for follow-up, only 5 of the 100 women recalled will have cancer [6]. In the case of False Negative, medical care is not sought in a timely manner. Treatment may even be delayed if cancerous symptoms are present. Understandably, the severity of the unintended consequences depend on the type and stage of the cancer.
It has been argued that screening may result in unnecessary earlier treatment or over-treatment. For breast cancer, the diagnosis and treatment of cancers that would otherwise never have caused symptoms or death in a woman’s lifetime can expose a woman to immediate risks. These include surgical deformity or toxicity from radiation therapy, hormone therapy, or chemotherapy, and late effects of therapeutic radiation – new cancers, scarring, cardiac toxicity.
Timely screening has proven to be an effective tool in curtailing cancer mortality rates. With early screening and engagement, health professionals are able to better tailor treatment plans to the individual tumor characteristics. Some screening tests and treatments are almost always recommended as the benefits outweigh the risks posed.
In our next article, we will explore the need for tools that facilitate the transition of care following a positive screening (False Positive and True Positive) through diagnostic, workup, and treatment plan. We will also elaborate further on directly engaging oncologists and the care team.
Written by Dorri Mahdaviani , who holds a Masters of Public Health (MPH) from the University of British Columbia (UBC). Her academic and professional interests include the areas of chronic illnesses, health care systems and childhood health and development.
Infographics and Design by Ann Wong, who holds a PhD in Biochemistry and Molecular Biology from the Faculty of Medicine at the University of British Columbia (UBC), Canada. She is an author of over 10 SCI publications, having taught at UBC and the Peking University Health Science Center (PUHSC) in Beijing.